 BER research complementary to the Genomic Science Program
BER research complementary to the Genomic Science Program
The BER structural biology and imaging resources—together with systems biology approaches—enable scientists to understand the relationships between plant and microbial genomes, protein structure and function, and environmental interactions important for bioenergy and environmental missions. A variety of techniques, many of which are available only at DOE User facilities, are available and can be used to probe target sizes of less than an Angstrom to millimeters across a range of temporal scales.
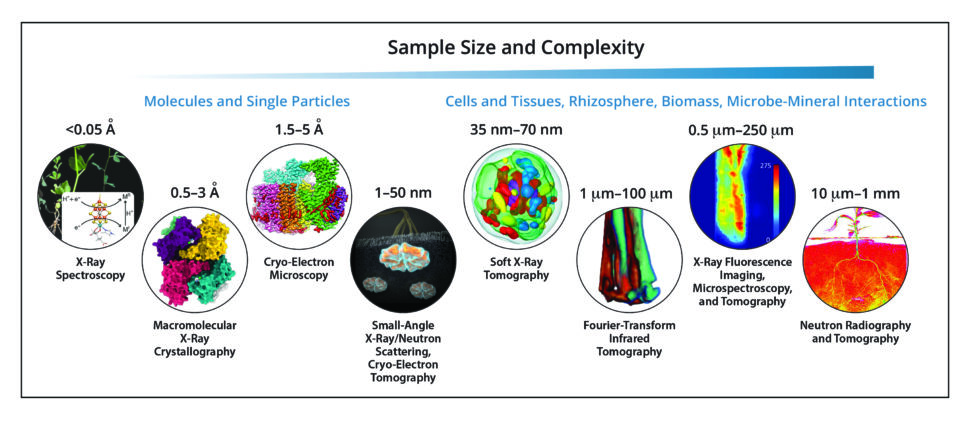
Structural Biology Techniques Address Sample Diversity. Critical structures and functions in biology occur across a wide range of distances (subnanometers to centimeters) and times (subpicoseconds to minutes). The DOE Office of Biological and Environmental Research makes available a variety of structural biology techniques suited to investigating different sample lengths and experimental time scales. [See bottom of page for image credits.]
User Experiences
Stories from scientists who used BER structural biology resources in their research.

Breaking Boundaries in IR Imaging to Explore Arctic Microbial Activity
July 28, 2022 – Neslihan Taş uses synchrotron-based infrared imaging to investigate shifting microbial processes in melting permafrost soils. More >

Filling the Nanoscale Gap in Understanding Wood Vulnerability
February 16, 2022 – Nayomi Plaza uses small angle neutron scattering at ORNL’s Center for Structural Molecular Biology to investigate wood durability. More >

Neutrons—Making Sustainable Biofuels
July 2, 2018 – Scientists at Oak Ridge National Laboratory are using neutrons to understand why certain hydrocarbons produced by blue-green algae are important to their biology. More >
More Information
- Website: https://berstructuralbioportal.org
- DOE Program Manager: Amy Swain, Amy.Swain@science.doe.gov
Image Credits
Composite image courtesy of Stanford Synchrotron Radiation Lightsource at SLAC National Accelerator Laboratory. Individual images left to right: (1) Van Stappen, C., et al. 2019. “Spectroscopic Description of the E1 State of Mo Nitrogenase Based on Mo and Fe X-Ray Absorption and Mössbauer Studies,” Inorganic Chemistry 58(18), 12365–376. DOI:10.1021/acs.inorgchem.9b01951. Reprinted under a Creative Commons License (CC BY 4.0). (2) Kim, Y., et al. 2021. “Tipiracil Binds to Uridine Site and Inhibits Nsp15 Endoribonuclease NendoU from SARS-CoV-2,” Communications Biology 4, 193. DOI:10.1038/s42003-021-01735-9. Reprinted under a Creative Commons License (CC BY 4.0). (3) PDB ID: 6MOR. Roh, S. H., et al. 2020. “Cryo-EM and MD Infer Water-Mediated Proton Transport and Autoinhibition Mechanisms of Vo Complex,” Science Advances 6(41), eabb9605. DOI:10.1126/sciadv.abb9605. (4) Courtesy Thomas SpleIstoesser, www.scistyle.com. See also Vandavasi, V. G., et al. 2016. “A Structural Study of CESA1 Catalytic Domain of Arabidopsis Cellulose Synthesis Complex: Evidence for CESA Trimers,” Plant Physiology 170(1), 123–35. DOI:0.1104/pp.15.01356. (5) Reprinted with permission from Roth, M. S., et al. 2017. “Chromosome-Level Genome Assembly and Transcriptome of the Green Alga Chromochloris zofingiensis Illuminates Astaxanthin Production,” Proceedings of the National Academy of Sciences USA 114(21), E4296– 4305. DOI:10.1073/pnas.1619928114. (6) Martin, M. C., et al. 2013. “3D Spectral Imaging with Synchrotron Fourier Transform Infrared Spectro-Microtomography,” Nature Methods 10, 861–64. DOI:10.1038/nmeth.2596. (7) Seyfferth, A. L, et al. 2017. “Evidence for the Root-Uptake of Arsenite at Lateral Root Junctions and Root Apices in Rice (Oryza sativa L.),” Soils 1(1), 3. DOI:10.3390/soils1010003. Reprinted under a Creative Commons License (CC BY 4.0). (8) Courtesy Oak Ridge National Laboratory. See also Dhiman, I., et al. 2017. “Quantifying Root Water Extraction After Drought Recovery Using sub-mm In Situ Empirical Data,” Plant Soil 417, 1–17. DOI:10.1007/s11104-017-3408-5.
