Biofuels Disruption of Membrane Domains: An Unrecognized Mode of Solvent Stress
Authors:
Jonathan D. Nickels1, Luoxi Tan1, Micholas Dean Smith2,3, Haden L. Scott3, Sai Venkatesh Pingali3, Hugh M. O’Neill3, Jennifer Morrell-Falvey3, John Katsaras3, Jeremy C. Smith2,3, James G. Elkins3* (elkinsjg@ornl.gov), and Brian H. Davison3
Institutions:
1University of Cincinnati; 2University of Tennessee–Knoxville; and 3Oak Ridge National Laboratory (ORNL)
URLs:
Goals
The Solvent Disruption of Biomass and Biomembranes Science Focus Area (SFA) provides fundamental knowledge about how solvents alter the structures of plant cell walls and microbial membranes. The project’s overarching hypothesis is that knowledge of partitioning or binding of the solvent from the bulk phase to biomass or biomembranes can help predict maximal or minimal disruption. Solvents disrupt biological structures comprising amphiphilic molecules and polymers (e.g., membranes and biomass). Determining common biophysical principles of solvent disruption will lead to new understandings of how solvents affect the relevant structures. This information will help determine the ultimate microbial limits in tolerating specific solvents, as well as the eventual design of co-solvents best suited for pretreatment. The SFA will integrate the power of world-class neutron scattering capabilities and leadership-class supercomputing facilities available at ORNL. These capabilities are complemented by expertise in biodeuteration and biomembranes at ORNL, plant cell wall chemistry at the University of Tennessee, and interpreting small-angle neutron scattering (SANS) data at the University of Cincinnati.
Abstract
A sustainable bioeconomy will undoubtably rely on the efficient production of lignocellulosic biofuels that can be combusted directly in automobile engines or catalytically upgraded to long-chain hydrocarbons for use as diesel and aviation fuels. Typical target biofuels include ethanol and n-/isobutanol which act as amphiphilic co-solvents in the aqueous environment of fermentation. Amphiphilic alcohols are well known to have chaotropic effects on biological molecules which lends to their inherent toxicity to the microbial biocatalysts used for fermentation. While these toxic effects can be broad, targeting all biological macromolecules, the cellular membrane is recognized as especially vulnerable to disruption due to the partitioning of amphiphilic co-solvents into the lipid bilayer. Functionally, this leads to membrane thinning, destabilization, loss of membrane potential, and eventually, cell death.
While the impact on the transverse structure of the membrane from high co-solvent titers is known, the effects on lateral biomembrane structure have not been well-studied. Lateral membrane structure is described as differences in lipid composition and membrane physical properties across the plane of the membrane. This can be understood in analogy to an in-plane phase separation which is dictated by the presence of high and low melting point lipid species as well as sterols (or their microbial analogs). Depending on the ratio of these molecules, phase separation occurs in the membrane, creating regions of different local composition and physical properties. In the biological context, these structures are colloquially known as membrane microdomains or lipid rafts. Rafts play an important role in many cellular functions due to their role as platforms to segregate and organize membrane proteins; this co-localization is critical to oligomerization of membrane proteins, and by extension, optimal cellular function.
In this project, researchers pursue the hypothesis that amphiphilic co-solvents, such as ethanol and n-/isobutanol, alter or disrupt functional membrane microdomains, leading to an unrecognized mode of co-solvent toxicity and cellular stress at non-lethal co-solvent concentrations. The team examines this hypothesis in a range of model and in vivo lipid membrane systems including phase-separating membrane mimics in the form of unilamellar vesicles, microbial membrane extracts, and engineered microbial systems that allow tunable membrane compositions. Domain structure and behavior is examined with a variety of non-destructive techniques including microscopy and SANS with and without the solvent ethanol (See figure). Computational approaches including molecular dynamics simulations (MD) are also leveraged to provide molecular detail and experimentally inaccessible understanding of membrane behavior in the presence of co-solvents.
Tan et al. (2023) has made a significant step forward in validating the SFA’s hypothesis by (1) demonstrating a direct disruption of a model lipid raft due to the presence of an amphiphilic co-solvent (ethanol) and (2) elucidating the physical mechanism by which the disruption of the lipid domains occurred. The team shows that unequal partitioning of ethanol between the co-existing phases leads to an increase hydrophobic mismatch of the thickness of these phases and a corresponding increase in the domain line tension. This is a driver to minimize the domain interface to domain area ratio. This represents the physical basis for a novel mode of co-solvent induced cell stress due to domain disruption. Continuing work will further test the hypothesis using more complex sample types, including live cells, and compare the effects of other amphiphilic biofuel molecules on domain organization. Further validation of the SFA’s hypothesis will provide a more holistic understanding of solvent-membrane interactions and inform actionable approaches to mitigating toxicity and improving biofuel yields from fermentative microbes.
Image
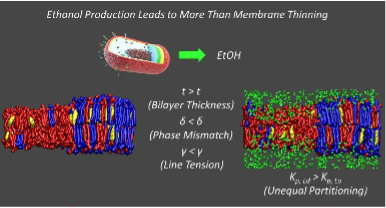
Figure. The Science Focus Area presents the hypothesis that membrane lateral organization is disrupted by amphiphilic co-solvents at concentrations lower than those which lead to full membrane destabilization. Left: a membrane composed of two lipids (blue and red) segregates into thinner and thicker domains. Right: When ethanol (green) is added it differentially partitions, thins the membrane and disrupts the lateral structure. Research shows precisely this type of disruption in model membranes and elucidates the mechanism by which it is occurring. This represents an unrecognized mode of solvent-induced stress in biofuel production. These findings establish new targets for intervention to improve fermentation yields (from Tan et al. 2023).
References
Tan, L., et al. 2023. “Amphiphilic Co-Solvents Modulate Structure of Membrane Domains,” ACS Sustainable Chemistry and Engineering 11(4), 1598–609. DOI:10.1021/acssuschemeng.2c06876.
Funding Information
This research is supported by the U.S. Department of Energy, Office of Science, through the Genomic Science program, Biological and Environmental Research (BER) Program, under FWP ERKP752. Oak Ridge National Laboratory is managed by UT-Battelle, LLC for the U.S. Department of Energy under contract no. DE-AC05-00OR22725.
