06/14/2022
Oleaginous Yeast Metabolically Engineered to Overproduce Promising Platform Chemical
Rhodotorula toruloides demonstrates potential as a host for triacetic acid lactone production
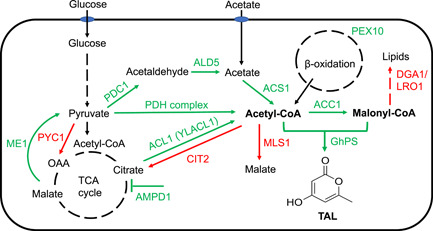
Metabolic pathway engineering strategies of Rhodotorula toruloides for biosynthesis of triacetic acid lactone (TAL). [ACC1, acetyl‐CoA carboxylase; ACL1, ATP‐citrate lyase; ACS1, acetyl‐CoA synthetase; PDC1, pyruvate decarboxylase; ALD5, acetylaldehyde dehydrogenase; AMPD1, AMP deaminase; CIT2, peroxisomal citrate synthase; DGA1, diacylglycerol acyltransferase; GhPS, 2‐pyrone synthase from Gerbera hybrida; LRO1, lecithin cholesterol acyltransferase; ME1, malic enzyme; MLS1, cytosolic malate synthase; PDH, pyruvate dehydrogenase; PEX10, peroxisomal matrix protein; PYC1, pyruvate carboxylase; YLACL1, ATP‐citrate lyase from Yarrowia lipolytica.]
[Reprinted under a Creative Commons license (CC BY 4.0) from Cao, M., et al. 2022. DOI:10.1101/2022.02.24.481788]
The Science
Rising demand for renewable biobased products has inspired research into using microbial cells as factories in place of traditional petroleum‐based chemical manufacturing processes. The oleaginous, or oil-producing, yeast Rhodotorula toruloides is one such promising host for synthesizing chemicals from biomass. Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) researchers metabolically engineered R. toruloides to produce increased levels of acetyl coenzyme A (acetyl-CoA) and malonyl-CoA during lignocellulosic breakdown, precursors to valuable chemicals such as triacetic acid lactone (TAL) which can be converted to a variety of valuable intermediates and end products such as phloroglucinol, acetylacetone, and sorbic acid.
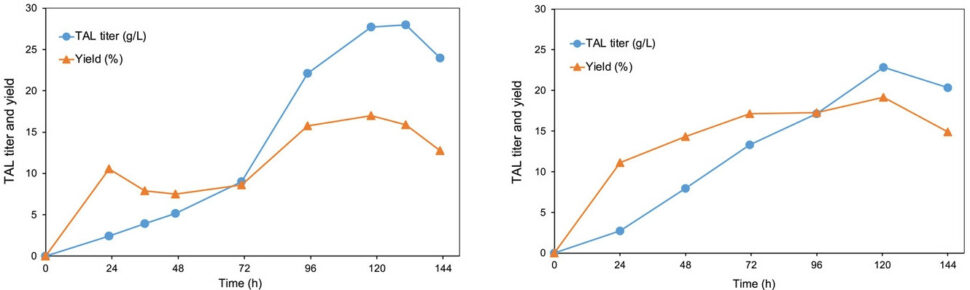
Fed-batch bioreactor fermentations of Rhodotorula toruloides for biosynthesis of triacetic acid lactone (TAL). The fermentation plots show TAL titer and corresponding yield on a glucose substrate (left) and an oilcane juice substrate (right). [Reprinted under a Creative Commons license (CC BY 4.0) from Cao, M., et al. 2022. DOI:10.1101/2022.02.24.481788]
The Impact
This work demonstrates that R. toruloides is a promising host to produce TAL and other acetyl-CoA-derived chemicals from renewable biomass.
Summary
Researchers screened several sources of the TAL-catalyzing gene 2-pyrone synthase (2-PS) and chose the version from Gerbera hybrida to functionally express in R. toruloides. Next, they systematically evaluated various metabolic targets in R. toruloides to increase its production of acetyl-CoA and malonyl CoA, with the intent of increasing TAL production. Sequential overexpression of the key pathway metabolic targets increased TAL production significantly in fed-batch bioreactor fermentations, increasing TAL production from 2g/L in a culture tube to 28 g/L from glucose and 23 g/L from oilcane juice.
Principal Investigator
Huimin Zhao
University of Illinois at Urbana‐Champaign
zhao5@illinois.edu
BER Program Manager
Shing Kwok
U.S. Department of Energy, Biological and Environmental Research (SC-33)
Biological Systems Science Division
shing.kwok@science.doe.gov
Funding
This work was supported by the Center for Bioenergy and Bioproducts Innovation, DOE Office of Science, Biological and Environmental Research program under award DE-SC00 18420.
References
Cao, M., et al. 2022. “Metabolic Engineering of Oleaginous Yeast Rhodotorula toruloides for Overproduction of Triacetic Acid Lactone,” Biotechnology and Bioengineering 119(9), 2529–40. DOI:10.1101/2022.02.24.481788.
