Optical and X-ray Multimodal-Hybrid Microscope Systems for Imaging of Plant Stress Response and Microbial Interactions
Authors:
Dara Dowlatshahi1,2* (daradow@slac.stanford.edu) Adam Bowman2, Rose Knight1,2, James Safranek1, Johanna Weker1, Jacob Summers2, Mary Beth Mudgett2, Federica Brandizzi3, Mark Kasevich2, and Soichi Wakatsuki1,2
Institutions:
1SLAC National Accelerator Laboratory; 2Stanford University; and 3Michigan State University
Goals
Development of next-generation correlative X-ray, light, and electron tomography by incorporating caged fluorescent protein and metal nanocrystals as both tracers for X-ray imaging/microscopy, electro-optical fluorescence lifetime imaging microscopy (EO- FLIM) and fiducial markers for Cryogenic electron tomography (Cryo-ET; See Fig. 1a). The initial application will be to study plant-bacterial pathogen interaction at the plant cell surface and transport of vesicles. In this early phase of development, researchers are highlighting plans and progress for a high frequency EO-FLIM setup at Stanford Synchrotron Radiation Lightsource (SLAC), caging of fluorescent fusion proteins for conjugation to metal nanocrystals with cage protein, and establishing plant systems to monitor membrane trafficking and transport.
Abstract
X-ray-FLIM coupling. The team’s current EO-FLIM configuration uses 40 and 80 MHz resonant gating (Bowman et al. 2019, Bowman and Kasevich 2021). The plan is to double this to 158.8 MHz in wide-field mode at SSRL X- ray imaging beamline BL 6-2. Some of the accomplishments in this area include: (1) sourcing of components for a new FLIM microscope to be integrated in X-ray microscopy beamlines for hybrid, X-ray pulse locked FLIM (See Fig. 1b) or used as a stand-alone instrument; (2) Testing the timing patterns at 158.72 Mhz with uniform fill (124 bunches) on the SSRL synchrotron on machine safety and electron stability; (3) Developing alternative approaches to produce X-ray excitation “single” pulses using a novel X-ray chopper crystal monochromator to select individual X-ray pulses from 476.3 MHz using a spinning silicon crystal at 75,000 rpm.
Novel biomarkers for multimodal imaging. The team’s previous work demonstrated the fusion of small proteins to the surface of a protein cage-like structure for cryo-EM structural determination of small biomolecules referred to as the double-shell system (Zhang et al. 2022). Modeling supported the feasibility to modify the existing system to encapsulate cargo for example having an external affibody and encapsulate a fluorescent protein within the protein cage that leaves headroom for conjugation of metal-based nanocrystals (MNCs) approximately 3-5 nm in size that emit X-ray photoluminescence with compatible short lifetimes (See Fig. 1a).
Some key accomplishments in this area: (1) refining modeling work in alpha-fold; (2) design of protein expression constructions and developing of purification strategies; (3) Cryo-EM analysis of first generation caged fluorescent proteins (See Fig. 1c).
The Brandizzi group used Arabidopsis thaliana as model plant species to show that members of the VAMP-associated- proteins (VAPs) family, VAP27-1 and VAP27-3, play a critical role in determining the topology of endocytosis in plant cells (Stefano et al. 2018. ).The goal is to analyze these processes using protein cages (See Fig. 1d) for hybrid multimodal X-ray imaging, Cryo-ET, and super-resolution imaging approaches. While the team develops these systems, members share some progress on establishing plant systems: (1) Vap27-YFP reporter strains grown and maintained for EO-FLIM measurements; and (2) Design and generation of constructs for expression and purification of VAP27 proteins and mutants for in vitro characterization supporting future in vivo experiments.
Image
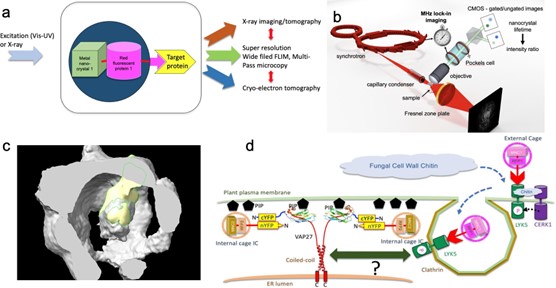
Figure 1. (a) Concept of novel combinatorial biomaker design for multimodal, multiscale bioimaging. (b) Hybrid imaging of plant-pathogen interactions using lock-in synchronization between X-ray photoluminescence, X-ray microscopy and FLIM. (c) Preliminary cryo-EM image of a protein cage with GFP at the center. (d) Design of combinatorial biomarkers for studying fungal-pathogen plant interaction through endocytosis and ER-plasma membrane contact site.
References
Bowman, A. J., et al. 2019. “Electro-Optic Imaging Enables Efficient Wide-Field Fluorescence Lifetime Microscopy,” Nature Communications 10(1), 1–8. DOI: 10.1038/s41467-019-12535-5.
Bowman, A. J. and Kasevich, M. A. 2021. “Resonant Electro-optic Imaging for Microscopy at Nanosecond Resolution,” ACS Nano 15(10), 16043–54. DOI:10.1021/acsnano.1c04470.
Stefano, G., et al. 2018. “Plant Endocytosis Requires the ER Membrane-Anchored Proteins VAP27-1 and VAP27-3,” Cell Rep. 23, 2299–2307.
Zhang, K. et al. 2022. “Cryo-EM, Protein Engineering, and Simulation Enable the Development of Peptide Therapeutics Against Acute Myeloid Leukemia,” ACS Cent. Sci. 8, 214- 222.
Funding Information
This program is supported by the U. S. Department of Energy, Office of Science, through the Biomolecular Characterization and Imaging Sciences Program, Office of Biological and Environmental Research, under BCIS FWP 100878.
