Engineering Synthetic Anaerobic Consortia Inspired by the Rumen for Biomass Breakdown and Conversion
Authors:
Elaina Blair1*, Roy Kim2, Joel Howard2, Patrick Leggieri1, Christopher Lawson2, Scott Baker3, and Michelle O’Malley1
Institutions:
1University of California–Santa Barbara, 2University of Toronto, Canada; and 3Pacific Northwest National Laboratory
Goals
This project will leverage a synthetic rumen consortium composed of anaerobic fungi and chain-elongating bacteria to study which metabolites are shared and exchanged between microbes and identify strategies to bolster lignocellulose conversion to value-added products. The project’s approach will develop high-throughput systems and synthetic biology approaches to realize stable synthetic consortia that route lignocellulosic carbon into short and medium chain fatty acids (SCFAs/MCFAs) rather than methane. Key research objectives are to (1) design and predict anaerobic fungal and bacterial consortia that efficiently convert lignocellulosic biomass into MCFAs, (2) understand how fermentation parameters and microbe-microbe interactions regulate and drive microbiome metabolic fluxes, and (3) use genomic editing to alter the fermentation byproducts of anaerobic fungi and bolster MCFA titers and yields.
Abstract
Lignocellulose deconstruction and conversion in nature is driven by mixed microbial partnerships rather than the action of a single microbe. For example, microbes are particularly well optimized to recycle organic matter in anaerobic habitats, ranging from landfills to intestinal tracts, via interspecies H2 transfer and methane release. Compared to aerobic processes, anaerobic digestion can be far more efficient in converting substrate to chemical products, largely because far less carbon is funneled to cell growth resulting in higher yields, and far less energy inputs are required because pre-treatment, aeration, mixing, and heat removal are greatly reduced. Compartmentalizing difficult biomass deconstruction and production steps among specialist anaerobes is an exciting new route to convert biomass into value-added products, especially if consortia can be built predictively and engineered for stability.
Previously, the team established a model bacterial consortium enriched from the rumen that converts lignocellulosic biomass into high titers of C4 volatile fatty acids (VFAs; butyrate) based on a chain elongation process that inhibits archaeal methanogenesis. Metagenomic and metatranscriptomic analysis identified key chain-elongating bacteria in these consortia that maintain high expression of the reverse β-oxidation pathway responsible for C4-C8 VFA production. This analysis also revealed several other bacterial species in the consortia that compete with chain elongators and reduce overall C4-C8 VFA yields by diverting carbon to unwanted products. Therefore, building synthetic consortia that eliminate these competing bacteria would bolster product yields and enable great control over VFA chain length. In parallel, the team also demonstrated that anaerobic rumen fungi within the Neocallimastix genus are superior biomass degraders compared to anaerobic bacteria from these enrichments. Moreover, the biomass degradation products lactate, acetate, and ethanol from Neocallimastix are optimal substrates for chain elongators.
Accordingly, partnering anaerobic fungi and chain-elongating bacteria in synthetic consortia represents a novel strategy for maximizing lignocellulose conversion to C4-C8 VFAs (Fig. 1).
Recently, the team screened multiple chain-elongating bacteria and identified candidates that grow robustly in culture media with known fungal metabolites and produce VFAs. Two of these strains are Megasphaera elsdenii and Pseudoramibacter alactolyticus. The team paired Neocallimastix spp. with each of these strains and cultivated them for numerous passages on reed canary grass. These cocultures show promise in terms of stability because both members were present after multiple transfers. Lactate is the key metabolic intermediate in these consortia, where the fungi make lactate as they degrade grass, and the chain elongators produce VFAs from lactate. High-performance liquid chromatography measurements showed that lactate from fungal cultures was consumed after chain elongators were added and butyrate was produced. Future plans include optimizing inoculation ratios of the different strains in consortia to maximize stability and VFA production. Researchers will employ qPCR to evaluate abundances of the community members over time and thus elucidate insight into the culture’s stability. Here, researchers further describe efforts to systematically characterize and model the production of VFAs from synthetic bacterial and fungal communities that have been grown on representative lignocellulosic grass substrates.
Image
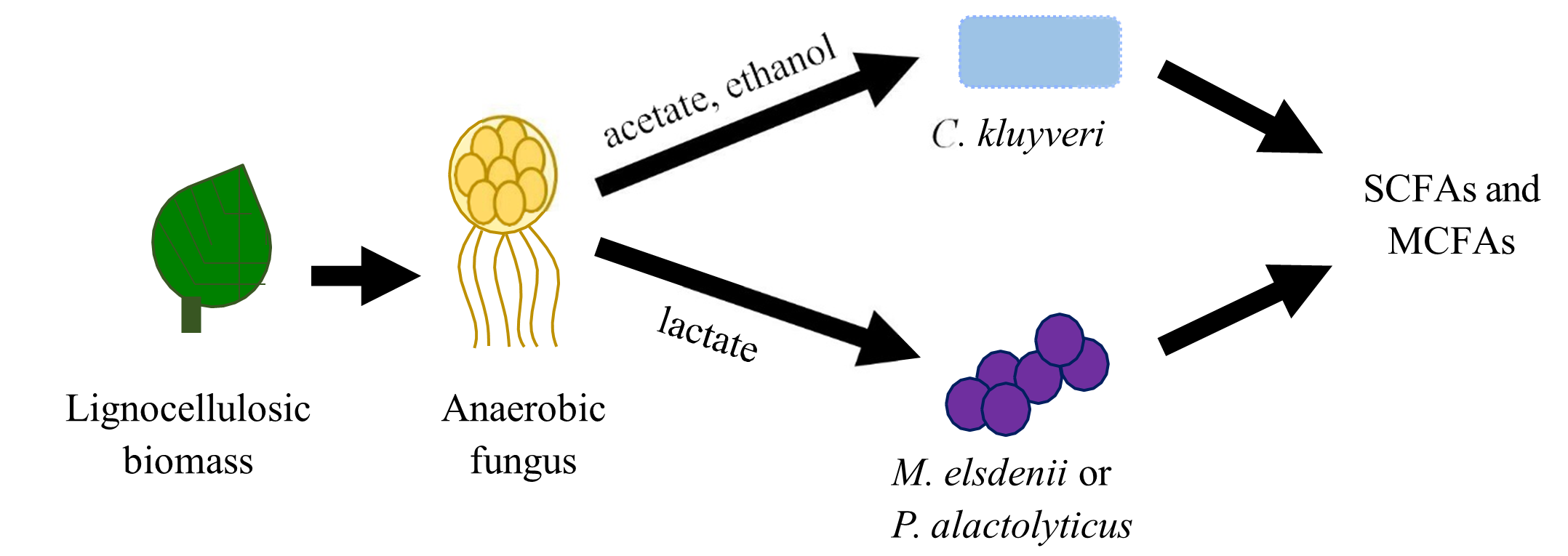
Figure 1. The flow of metabolites during lignocellulose degradation and conversion to VFAs provides the guiding principle for designing synthetic anaerobic consortia.
Funding Information
This research was supported by the DOE Office of Science, Office of Biological and Environmental Research (BER), grant no. DE-SC0022142.
